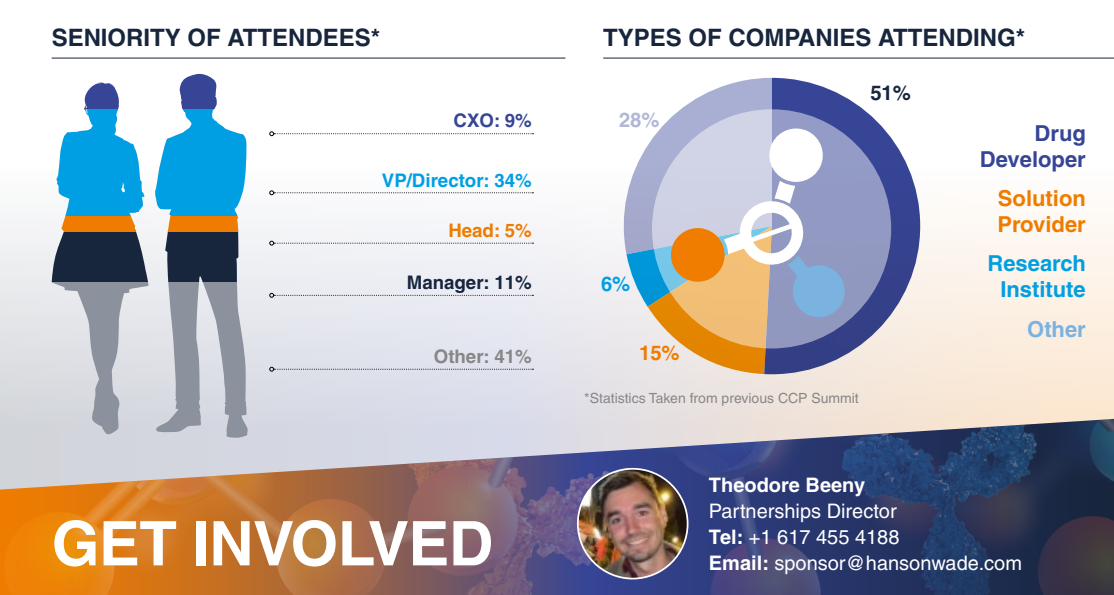
The 9th CCP Summit was the only industry-led meeting extending across varying modalities, with senior leadership attendees and a new application angle. Given that the biopharma industry is somewhat rooted from traditional manufacturing methods, finding the most relevant solution providers who are geared towards continuous approaches remains a difficulty. At the same time, drug development must continue, evident as we see companies such as Eli Lilly and Novo Nordisk expand their sites for pipeline progression.
Therefore, at a time where sustainability measures and capital efficiency is key, this was your unique forum to establish yourself as the hero for continuous processing applications and shed light on your relevance amongst the dream market.
Experts Needed Your Help With:
CDMO capabilities to take the weight of scale-up transitioning off their shoulders
Small molecule and biologics continuous processing equipment to enable in-house development with minimal downtime

PAT and analytical software to help maintain the gold standard of product quality

Facility design services to support biopharma in their shift to continuous
Why Partner?


Establish your solutions ahead of 60+ process development and CMC senior professionals across pharma and biotech to appeal to your services
Communicate your service flexibility and specificity to shed light on your critical solutions
Engage in a 3-day networking and interactive opportunity to understand must-have continuous processing demands
Participate industry-focused meeting targeting any and every process development expert for all modalities!